SCIENTIFIC PROGRAMProgram (updated september 19, 2019
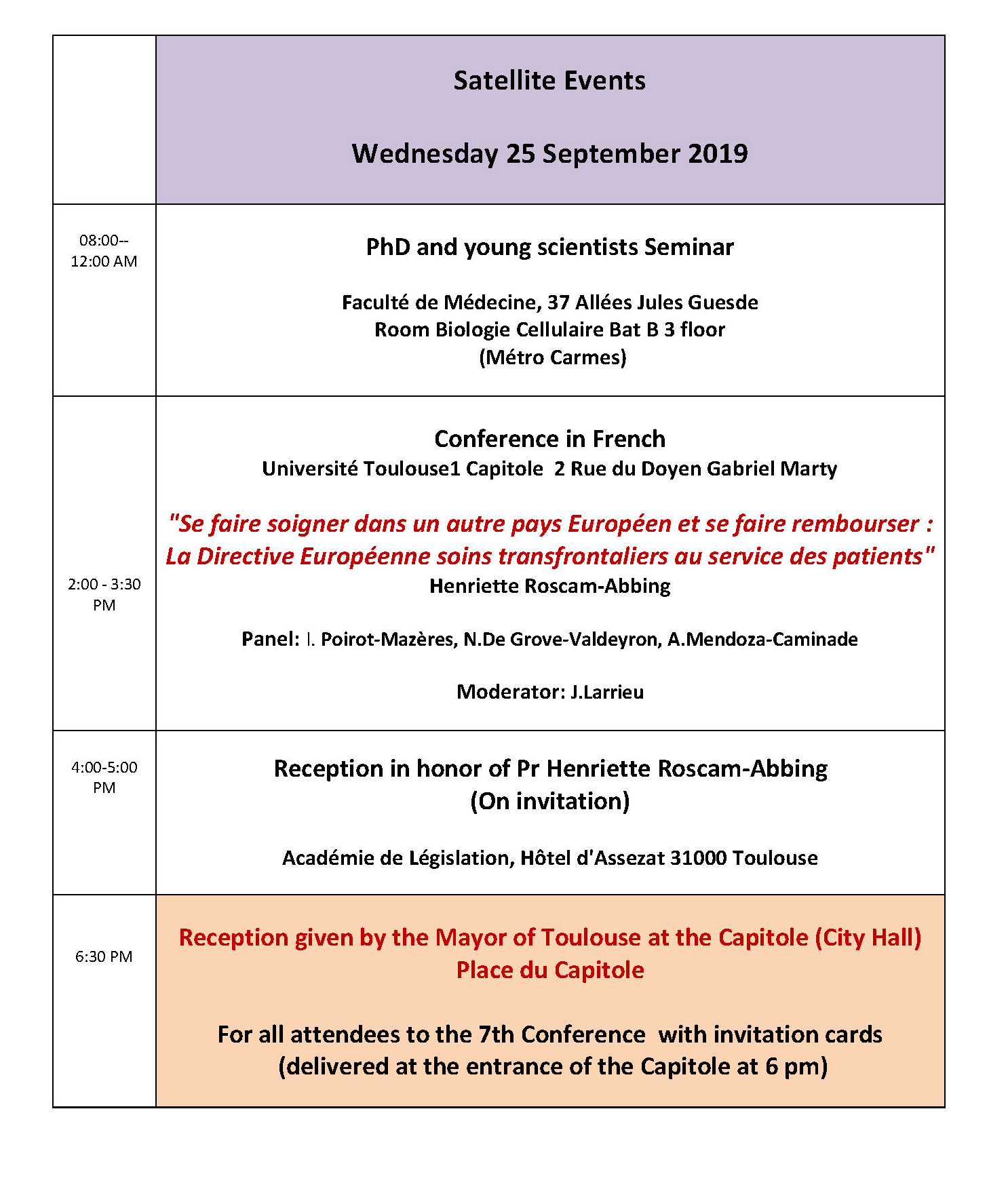
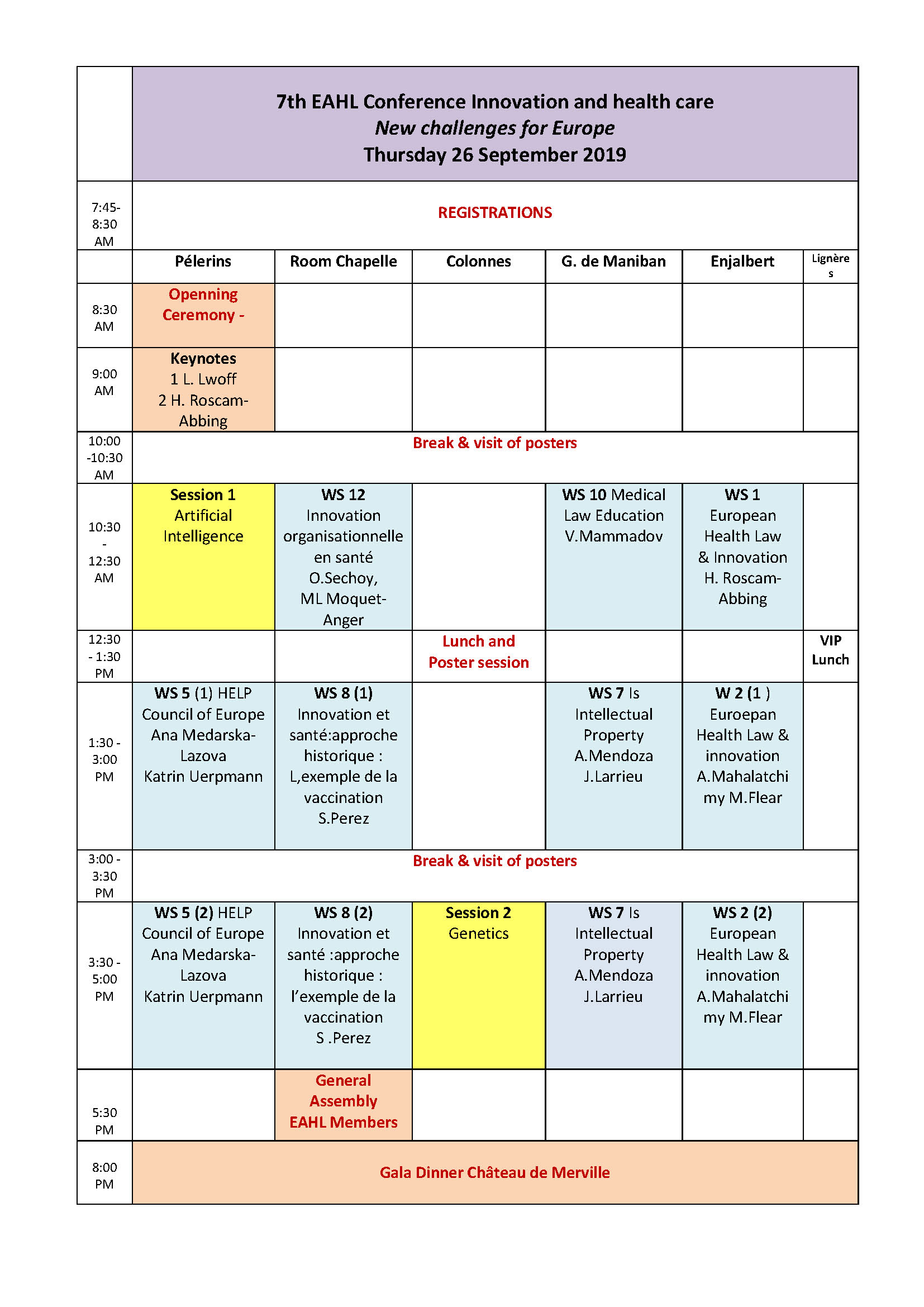
Innovation and Health Care New Challenges for Europe7th EAHL Conference Toulouse25 September 2019 Faculté de Médecine, 37 allées Jules Guesde Room Biologie Cellulaire Bat B 3rd Floor (Métro Carmes) 8:00-12:00 PhD and young scientists Seminar Oral presentations Jury: Emmanuelle Rial-Sebbag (France) , Henriette Roscam-Abbing (The Netherlands), Athanasios Panagiotou (Greece). PHD O1 New technologies in the courtroom : can justice keep up with biotechnological advancements? Mirko Dukovic Central European University (CEU PHD O2 E-Health issues in European Union. law Nahela El Biad (Université Aix Marseille) PHD O3 Vulnerable people and clinical trials: Reflections in European law. Eloïse Gennet (Post-doctoral researcher, INSERM U1027, Toulouse). PHD 04 Personalized Medicine, Priority Setting and Regulation: Consolidating equal access, cost-effectiveness and the development of new technologies Kaisa-Maria Kimmel, University of Lapland PHD O5 Uterus transplantation: a legal transplant? Lina Oplinus (KU Leuven) PHD O6 The use of unproven stem cell therapies in clinics: Can it be limited? Delphine Pichereau Inserm UMR 1027,Université Paul Sabatier, Toulouse, France PHD O7 Adaptive regulatory pathways for medical products Mathijs van Westendorp (Ku Leuven Belgium) Eroom’s law Université Toulouse1 Capitole 2 Rue du Doyen Gabriel Marty 2:00 -3:30 pm Conference Salle Gabriel Marty "To receive healthcare in any other European Union country and to be reimbursed: The European Directive on cross border healthcare at the service of European patients" Speaker Henriette Roscam-Abbing Moderator Jacques Larrieu Académie de Législation, Hôtel d'Assezat 31000 Toulouse (On invitation) 4:00-5:30 pm Reception in honor of Pr Henriette Roscam-Abbing Capitole (City Hall) Place du Capitole 6:30-8:00 pm Reception given by the Mayor of Toulouse for all attendees. NB For security reasons only registered attendees can access with an invitation card that will be delivered at the entrance of the Capitole at 18:00. Thursday 26 September 2019Hotel Dieu Room Pélerins 8:30 am Opening session Chairperson: Karl Harald Sovig (University of Bergen Norway) Introductive talk by Karl Sovig President of the European Association of Health Law Welcome talk by Marc Penaud General Director of the Toulouse University Hospital Message from Mrs Irene Norstedt Head of Unit, Health Directorate General for Research and Innnovation, European Commission 9:15 am Key note speakers Chairperson : Karl Harald Sovig (University of Bergen Norway) K1 Laurence Lwoff Conseil de l'Europe Direction des droits de l'Homme, Unité de Bioéthique New Technologies : new challenges for human rights ? The work of the Council of Europe K2 Prof. dr. Henriette Roscam Abbing European Journal of Health Law Innovative technologies, a challenge for equal access to health care in Europe 10:30 am Session 1 Artificial intelligence Chair persons: Florence Sedes (University Paul Sabatier France) & Tom Goffin (University of Ghent Belgium) O1 Patient rights for a trustworthy Artificial Intelligence Tom Goffin University of Ghent O2 Key legal issues around the use of Artificial Intelligence in medical technology Monika Kupis Jagiellonian University [Krakow] (UJ) O4 Artificial intelligence and professional liability Tom Balthazar Ghent University [Belgium] (UGENT) O5 The standard of due diligence in using algorithms in medicine Tomasz Sroka Department of Bioethics and Medical Law, Jagiellonian University, Krakow O6 Artificial Intelligence applied to diagnosis and treatment: facing legal and ethical challenges of big data and robotics in healthcare. Joaquin Cayon-De Las Cuevas IDIVAL-University of Cantabria Spain 1:30 -5:00 pm Workshop 5 Council of Europe The Council of Europe Education programme on Human rights (HELP) and bioethics Coordinators: Ana Medarska-Lazova, HELP Project Officer (CoE); Katrin Uerpmann, Administrator, Bioethics Unit (CoE); Chairpersons: Laurence Lwoff, Head of Bioethics Unit, CoE Annagrazia Altavilla, European Association of Health Law Board of Directors – France Representative - Espace de Réflexion Ethique PACA-Corse Introduction O98 HELP courses and the HELP Methodology : HELP courses developed jointly by the CoE Bioethics and HELP Units Ana Medarska-Lazova (HELP Unit, CoE) O96 Key human rights principles in Biomedicine I (Introduction) Ivana Roagna,Attorney, Human Rights trainer, Asti – (Italy) O99 Key human rights principles in Biomedicine II (Free and Informed Consent, Medical Confidentiality) Katrin Uerpmann(Bioethics Unit, CoE) O97 The application of key human rights principles in Biomedicine in specific contexts (Biomedical research and genetic tests) Fabio Macioce, Professor of Legal Theory and Bioethics, LUMSA University, Rome – (Italy) O100 Data Protection and Privacy Rights Raluca Bercea, PhD, Lecturer at the Faculty of Law, Timisoara University – (Romania) Room Chapelle 10 :30 am Workshop 12 (in French) Innovation organisationnelle en santé Coordination O.Sechoy, Marie-Laure Moquet-Anger. Direction de la recherche CHU Toulouse et Association Française de droit de la santé O112 Educ@dom, dispositif de télésurveillance et télé-éducation Marie Christine Turnin, Jacqueline Delaunay, Helene Hanaire, Pierre Gourdy CHU Toulouse O113 L’Institut Toulousain de simulation en Santé (It-SimS), Vecteur d’innovation en pédagogie et en santé Pr Thomas GEERAERTS CHU Toulouse O114 Innovation organisationnelle en santé : le dispositif de soins partagés en psychiatrie Dr Sophie Prebois CHU Toulouse 1:30-5:00 pm Workshop 8 (in French) Innovation et santé: approche historique: l’exemple de la vaccination au travers de l’histoire du droit et de l’histoire de la médecine Coordinateurs Stanis Perez O102 Du vivant dans le vivant pour le vivanT. Retour sur la généalogie de la vaccination dans l'histoire de la médecine à l'âge classique. Stanis Perez Pléiade Université Paris 13 : EA7338 Société française et francophone d'éthique médicale (SFFEM) AIEMPP O103 La « pollution » du vivant par le vivant : les humeurs animales et la vaccine au XIXe s.Laurent-Henri Vignaud (Centre Georges Chevrier UMR 7366-Université de Bourgogne) O104 Quelques exemples des craintes contemporaines d’empoisonnement par les vaccins : mythes de la pollution par les vaccins vivants, par l’ADN ou les produits d’origine fœtale Françoise Salvadori (CIMEOS-Université de Bourgogne) O105 Politiques vaccinales européennes : disparités et sensibilités Annick Opinel Institut Pasteur de Paris 5:30 pm EAHL General Assembly of EAHL Members
Room Colonnes 10 :00-10 :30 am Coffee break and poster session 12:30- 1:30 pm Lunch and poster session 1:30-5:30 pm Session 2 Genetics Chair persons Anne Cambon-Thomsen ( URR INSERM 1027 Paul Sabatier France) & Yann Joly ( McGill University Canada) O07 Germline Gene Editing and the Challenges for an International Regulation (26) Noemi Conditi Alma Mater Studiorum University of Bologna (UNIBO) O08 Ethical and legal considerations of predictive genetic testing (30) Ingrid Soraya Alvarado Lopez University of Bologna (UNIBO) O09 Genome Editing, Ethics and the Convention on Human Rights and Biomedicine – Is the Time Ripe for Reassessment? (141) Amanda Blick, Juli Mansnérus University of Helsinki, Faculty of Law O10 Who is my patient? An exploration of the definition of the patient in medical law (111) Naomi Hawkins University of Exeter Law School , Daniele Carrieri University of Exeter Medical School O11 Protecting the Best Interests of the Child in the Regulation of Gene Editing Technologies (121) Andrea MulliganTriniy college Dublin2 O12 Consent for genome research on behalf of the child in early childhood (137) Merike Helander University of Helsinki 013 Stem cells in interaction with intellectual property law Brožová Sandra University of Economics, Prague
Room Gaspard de Maniban 10:30 am-1:00 pm Workshop 10 Medical law education: innovation, healthcare, Justice and multiculturalism (WAML education committee) Coordinator: Vugar Mammadov O109 Medical law Education : Innovations, Healthcare, Justice and Multiculturalism Vugar Mammadov WAML, Law Faculty of Baku State University (WAML, BSU) Azerbaijan Medical University (AMU) Iryna Senyuta Danylo Halytskyi Lviv National Medical University Maria Deliverska Sofia Medical University (SMU) O59 Some Issues on Special Legal Status of Siamese Twins as Patients Oksana Harasymiv Iryna Senyuta Danylo Halytsky Lviv National Medical University 2:00-5:00 pm Workshop 7 Is Intellectual property a booster or an hurdle for the health care innovation ? Alexandra Mendoza-Caminade and Jacques Larrieu Panel Pierrick Rousseau (Intellectual property Director Fabre SA) Florence Taboulet (Université Paul Sabatier) Elisabeth Berthet (Attorney ,Promark Paris) Charlotte Lamure (PhD Toulouse1 Capitole) Room Enjalbert 10:30 am Workshop 1 Joint workshop EJHL / EAHL Emerging health related (converging) technologies: a challenge for social justice and patient rights (Henriette Roscam Abbing) O77 Artificial Intelligence and Machine Learning in Medicine: Medical Liability Issues Athanasios Panagiotou Aristotle University of Thessaloniki, Faculty of Law, Laboratory for the Research of Medical Law and Bioethic O78 Doctors on line: a promise or a threat for social justice and patient rights? Titti Mattsson Faculty of Law Health Law Research Centre, Lund University O79 A child's right to enjoy the benefits of scientific progress and its applications in health care: a good deal? Santa Slokenberga Lund University [Lund] O80 Artificial intelligence, robotics and the potential legal issues concerning healthcare and patients' rights Brenda DalyAssociate Professor of Law Dublin City University (DCU) O81 Artificial Intelligence and automated decision-making in healthcare Ana Nordberg Lund University, Faculty of Law, Health law centre 2:00-5:00 pm Workshop 2 European Health Law and Innovation: substantive aspects and embedding in national legal orders Coordinators : Aurélie Mahalatchimy and Mark Flear This two-parts discussion-workshop will first explore the substantive aspects of European health law (the law of the EU, the CoE and the EPO) relating to innovation in healthcare, in particular its specificities, and second discuss the embedding of European health law within national legal orders and the potential need for the establishment of an EAHL interest group on this topic. Panel: Annagrazia Altavilla (Espace Ethique Paca-Corse), Estelle Brosset (University of Aix-Marseille), Joaquin Cayon De Las Cuevas (University of Cantabria), Nathalie De Grove-Valdeyron (University of Toulouse), Arnaud FARRUGIA (University of Paris-Nanterre), Eloïse Gennet (University of Toulouse), Nahela El Biad (University of Aix-Marseille), Naomi Hawkins (University of Exeter), Phoebe Li (University of Sussex), Jean McHale (University of Birmingham), Andrea Mulligan (Trinity College Dublin), Stefania Negri (University of Salerno), Emmanuelle Rial-Sebbag (University of Toulouse), Judit Sandor (Central European University Budapest), Tomislav Sokol (Croatia), Karl Sovig (University of Bergen). Friday 27 September 2019
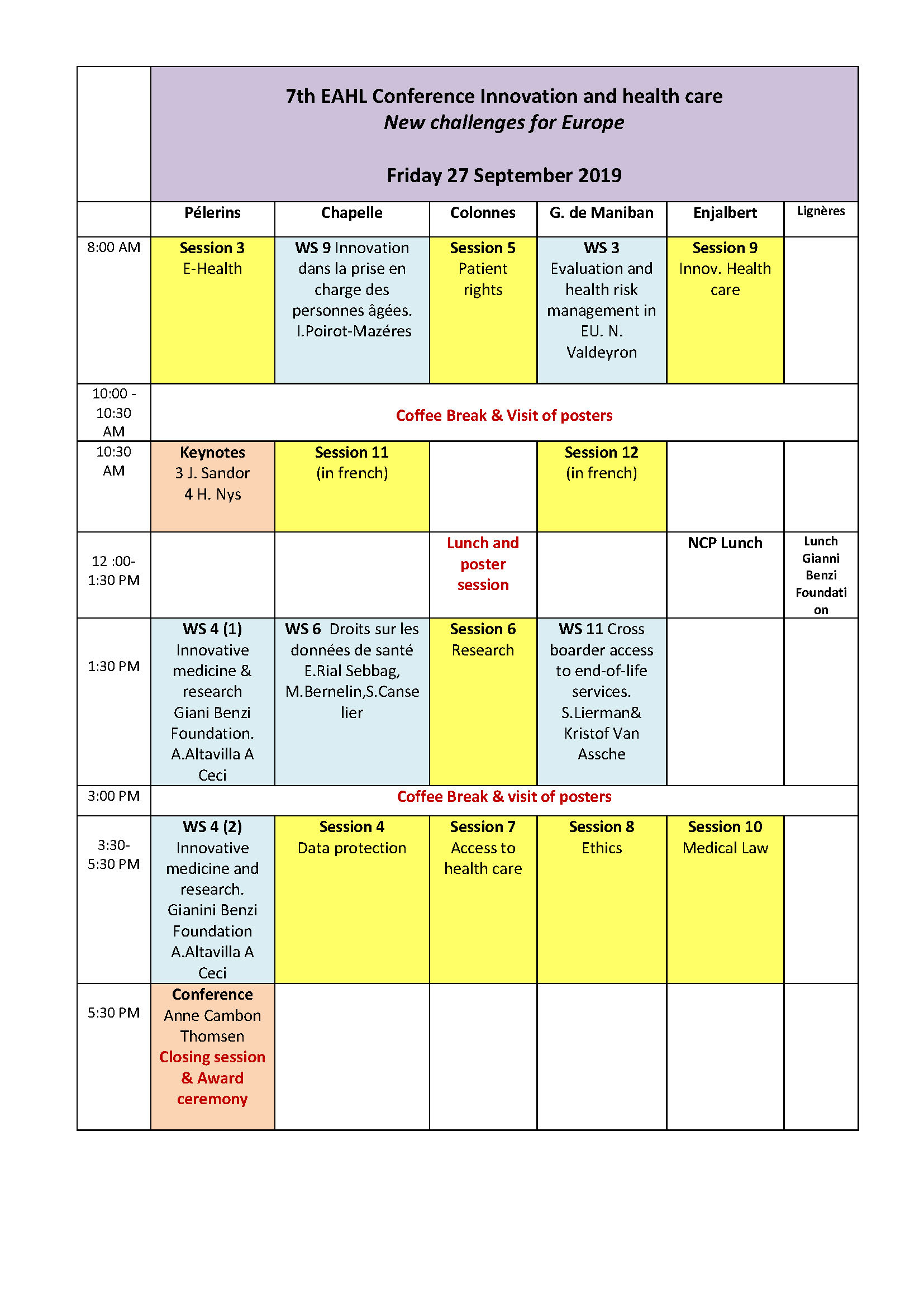
Room Pélerins 8:00 am Session 3 E-Health Chairpersons Fedele Bonifazi Gianni Benzi Foundation Italy & Iryna Senyuta (Ukraine) O14 Attitudes of Estonian Healthcare Professionals to Internet-delivered Cognitive Behavioural Therapy. Melita Sogomonjan Tanel Kerikmäe, Peeter Ross, Pille Ööpik, Tallinn University of Technology (TalTech) O15 Mobile health and data privacy challenges Klaudia Surmacz Jagiellonian University (UJ) Cancelled O16 Role of E-health at Healthcare Reform in Ukraine Ivan Demchenko Legislation Institute of Verkhovna Rada of Ukraine O17 Are well-being apps in good (e)health? Introducing the borderline problem in light of the new medical devices regulation István Böröcz, Audrey Van Scharen, Eugenio Mantovani . O18 Innovating health care by e-health: disruptive implications of new technologies Jaap Sijmons , Melita Van Der Mersch , Mirna Oosting Utrecht University [Utrecht] O19 Big data, personalized medicine: tensions between autonomy and solidarity in the welfare state Mette Hartlev, Katharina O Cathaoir Faculty of Law, University of Copenhagen 11:00 am Keynotes Chairperson Karl Harald Sovig (University of Bergen Norway) K3 Judit Sandor Professor Central European University Budapest Human Genome Editing: What are the Legal Implications for Europe and for the World? K4 Pr Herman Nys Catholic University of Leuven - Katholieke Universiteit Leuven (KU Leuven) The European Journal of health law 1994-2019 How innovative has been its contribution to the development of health law in Europe ? 1:30–4:30 pm Workshop 4 Innovative medicine and research: ethical, legal and regulatory issues sponsored by the Gianni Benzi Foundation Coordinators Annagrazia Altavilla – Adriana Ceci Welcome speeches : O85 Regulatory Science and Innovation: the role of Gianni Benzi Foundation for Pharmacological Research Adriana Ceci President of the Gianni Benzi Foundation for Pharmacological Resea O86 Innovative medicine and research: ethical, legal and regulatory issues Annagrazia ALTAVILLA Lawyer – Responsible of the International Relations of Espace Ethique Paca-Corse, AP-HM (France). Member of the Board of Directors of the EAHL. Laurence Lwoff, Head of the Bioethics Unit – Human Rights Directory - Council of Europe Introductory session O87 Models of Governance for Innovation in Medicine and Health Research (152) Siobhán O’Sullivan Royal College of Surgeons in Ireland Dublin, Ireland Chair of the COE DH-BIO Drafting Group on the Strategic Action Plan for 2020-2025, Vice-chair of the European Group on Ethics in Science & New Technologies Session I - Innovative medicine and research (Fondation BENZI) Chairman Timo Minssen, Director, Centre for Advanced Studies in Biomedical Innovation Law (CeBIL), University of Copenaghen, (Denmark) O88 Access to Personal Data for Scientific Research in the Perspective of Developing Innovative Medicines Jean Herveg CRID Head of LIS (Liberties & Information Society), University of Namur (Belgium), Coordinator of the EAHL “Data Protection law and policy WG” O89 Legal and Regulatory issues dealing with paediatric translational research in the EPTRI (European Paediatric Translational Research) framework Dr. Olga Tzortzatou, Biomedical Research Foundation of the Academy of Athens (Greece) O90 Intellectual Property Protection of Genetic Material and Information in the Pharmaceutical Sector? - Legal Questions on Genetic Material and Genome Sequencing between Innovation, Protection and Sharing Claudia Seitz : Faculty of Law, Center for Life Sciences Law (University of Basel) University of Basel - Suisse Session II - Access to health care and innovation Chairman Joaquin Cayon De Las Cuevas, University of Cantabria (Spain), Director of the Research Group on Health Law and Bioethics at IDIVAL, Member of the Board of Directors of EAHL O91 Health Technology Assessment (HTA) and access policies (81) Verena Stühlinger Private University for Health Sciences, Medical Informatics and Technology GmbH (UMIT) – Vice-President of the EAHL O92 Machine Learning Systems applied to health data Fedele Bonifazi Vice-President Fondazione per la Ricerca Farmacologica Gianni Benzi Onlus – (Italy) O93 Exploring solutions to foster ATMP development and access to patients in Europe Vincenzo Salvatore University of Insubria, (Italy) Former Head of the Legal Service and Data Protection Officer at the European Medicines Agency Session III - Empowerment and patients’ rights in innovative healthcare Chairman Tom Goffin, Ghent University (Belgium) O94 Orphan medicinal products and health budgets: our role as patient advocates François Houÿez European Organisation for Rare Diseases (EURORDIS), Representative at the European Network of HTA Agencies, and the European Medicines Agency O95 Health vulnerability and the European framework on access to orphan medicines (82) Eloïse Gennet INSERM (UMR 2017 Équipe 4) Discussion Chair persons E. Bonsone – SIARV President, Board of Directors member, Fondazione per la Ricierca Farmacologica Gianni Benzi Onius (Italy) & Annagrazia Altavilla Lawyer – Responsible of the International Relations of Espace Ethique Paca-Corse, AP-HM (France). Member of the Board of Directors of the EAHL. 5:30 pm Closing conference Chairperson Emmanuelle Rial-Sebbag (UMR INSERM 1027 UPS France) Conference : O115 Ethical dilemma in introducing innovative technologies in clinical practice: ethical framework and the place of legal instruments. Anne Cambon-Thomsen (France) Award ceremony for winners of PhD Seminar and of Conference posters Room Chapelle 8:00 am Workshop 9 (in French) Innovations dans la prise en charge numérique des personnnes âgées. Aspects techniques, éthiques et juridiques Coordinateur: Isabelle Poirot-Mazères O106 Gérontologie et e-santé : le droit à l'épreuve de l'innovation et de la protection des personnes âgées (83) Renaud Bouvet Faculté de médecine de Rennes Université de Rennes I CHU Rennes France O107 Les biomarqueurs numériques sont une étape nécessaire pour atteindre les objectifs proclammés de la médecine modern. Antoine Piau Gérontopole CHU Toulouse Orcatech team, OHSU Portland O108 Consumérisation de la santé et e-santé : vers la fin des acteurs traditionnels ? Le cas de la silver Economie (108) Yann Ferrari Toulouse 1 Capitole Centre Maurice Hauriou 10:45 am Session 11 (French) Chairpersons Bénédicte Boyer-Bévière (Université de Paris 8 France) & Nathalie De Grove Valdeyron ( Université Toulouse1 Capitole France) O71 Vers des politiques nutritionnelles innovantes? l'évolution du droit de l'Union européenne et du droit français en matière de prévention de l'obésité et du surpoids Marine Friant-Perrot Université de Nantes O66 L'importance d'une réflexion bioéthique internationale à l'égard des activités de recherches utilisant la méthode Crispr-Cas9 – L'exemple des bébés chinois génétiquement modifiés » Bénédicte Bévière-Boyer Université de Paris 8 O67 L’épineuse question des prix des médicaments innovants en EuropeTaboulet : quelles perspectives ? Florence Taboulet, Blandine Juillard-Condat O65 Enjeux éthiques et juridiques lors du rendu des résultats de séquençage génétique en pratique médicale Perrine Malzac, Marion Mathieu, François Faurisson (Espace Ethique Paca-Corse). O69 Implication des associations de malades dans processus décisionnel : pour une « participation informée » François Faurisson, Marion Mathieu,Perrine Malzac (Mission Inserm Association Comité d’Ethique Inserm). O70 Les médicaments innovants et le défi de la sécurité sanitaire Florence Taboulet Université Paul Sabatier Toulouse 3 - INSERM UMR 1027 Aurélie Mahalatchimy CNRS UMR 7318 DICE CERIC, CNRS-Aix-Marseille Université CNRS : UMR7318 1:30 pm Workshop 6 Droits des données de santé et des bases de données: quelles innovations juridiques?Coordinators Emmanuelle Rial-Sebbag, Margo Bernelin, Sonia Desmoulin-Canselier Panel: Danielle Bourcier (Centre d’études et de recherche de science administrative (Université Paris II Assas) Amandine Cayol (Institut Demolombe Université de Caen Normandie) , Emilie Debaets, Institut Maurice Hauriou, Université Toulouse1 Capitole).. A l’ère du Big data, où l’on traite un nombre toujours plus important de données, notamment en santé, des voix s’élèvent désormais pour demander à ce que les individus disposent d’un droit de propriété sur leurs données de sorte à ce qu’ils puissent tirer un bénéfice de l’exploitation commerciale de ces dernières. Partant, la production d’un modèle juridique alternatif est en discussion. Les questions sont alors nombreuses : est-il opportun d’élaborer un droit de propriété sur les données ? À quelles conditions et pour quelle efficacité en termes de protection des personnes ? Quels en seraient les inconvénients ? Quels autres mécanismes juridiques peuvent-être envisagés ? Ces questions seront au cœur de la session proposée. 3:30 pm Session 4 Data protection Chairpersons : Jean Herveg (CRID University of Namur Belgium) Herman Nys (KU Leuven) O21 Personal Health Data security and privacy protection in the e-health era Luciana Caenazzo, Pamela Tozzo University of Padua (Italy) (DMM-Padua University) O22 Data sharing in the context of learning healthcare systems: A common objective for patients, physicians and researchers Yann Joly McGill University O23 The Unofficial Apartheid – Scientific research for public and private bodies under the GDPR Paul Quinn Health and ageing lab Vrije Universiteit Brussel (VUB) (HAAL) O24 Human rights and postgenomic biometrics: protecting the public against potential misuses of personal epigenetic and microbiomic information (Charles Dupras Centre of Genomics and Policy [Montréal] (CGP) O26 Access to and Re-use of Government Data and the Use of Big Data in Healthcare Miet Caes Leuven Institute for Healthcare Policy (KU Leuven) (LIGB) Room Colonnes 8:00 am Session 5 Patient rights Chairpersons Christian Hervé (Université Paris Descartes France) & Steven Lierman (KU Leuven Belgium) O26 Healthcare Decision-Making and the Older Person in Ireland John Lombard University of Limerick O27 Does emergency rescue access to drug technologies save patients - assessment of Polish regulations / solutions Anna Jacek Faculty of Law and Administration (FLA Rzeszow) O28 The patient’s treatment plan in Slovenian health law Blaž Ivanc University of Ljubljana, Faculty of Health Sciences (ZF UL) O29 Patient Engagement Technology in the light of patient's right to information Zuzanna Zapotoczna Uniwersytet Jagielloński (UJ) O30 Worlds Apart or Two Sides of the Same Coin? Attitudes, Perceptions and Motives of Potential Oocyte and Sperm Donors in Austria Magdalena Flatscher-Thöni , Bettina Böttcher, Caroline Voithofer, Gabriele Werner-Felmayer, Astrid Lampe, Wilhelm Geser, Claudia Schusterschitz, UMIT - University for Health Science, Medical Informatics and Technology (UMIT) O31 Personalised medicine, medical law the rights of the patient Marta Sjenicic Institut of Social Sciences (ISS) O32 Patient Safety as a Human Right. Is Tort Law the Right Remedy? Mariya Sharkova Faculty of Public Health (FPH) 1:30 pm Session 6 Research Chairperons Judit Sandor ( Central European University Hungary) & Joaquin Cayon de Las Cuevas (University of Cantabria Spain) O33 The liminality of research ethics committees: navigating participant protection and research promotion Edward Dove School of Law, University of Edinburgh O34 Key Legal Challenges Associated with Biobanks: The Example of the International Biobank for the Collection, Cultivation and Dissemination of Inner Ear Organoids in the UK Maria Paraskeva DR PSOMA WELLNESS LTD O35 Review of medical devices legal regulation in Bulgaria and European Union Kremena Ivanova, Stamatios Priftis , Antoniya Yanakieva Faculty of Public Health, Medical University - Sofia (FPH, MUS) O37 Regulation and surcumstances in conduction of Clinical Trial Management education in Bulgaria Antoniya Yanakieva Assena Stoimenova , Kremena Ivanova Faculty of Public Health (Prof.) 2 : Faculty of Public Health (FPH) O39 The influence of the EU clinical trials legislation on the criminal law Malgorzata Galazka Université Catholique de Lublin Jean-Paul II 3:30 pm Session 7 -Access to health care and innovation Chairpersons Ofelia de Lorenzo (Spanish Association of Health Law Spain) & Vugar Mammadov (Azerbaijian Bakou State University) O41 Access to innovative unauthorized medicines under specific procedures - the perspective of Poland Katarzyna Melgies John Paul II Catholic University of Lublin (KUL) O42 Geographical equality in access to innovative healthcare Michal Koscik Faculty of Medicine, Masaryk University (LF MU) O43 A legal perspective on improving access, affordability and quality in healthcare: comparing the UK and the Netherlands Jos Boertjens Dutch Healthcare Authority (NZa) Mary Guy Lancaster University O44 Psychiatric Patients Requesting Euthanasia: Initiatives for Sound Clinical and Ethical Decision Making Verhofstadt Monica Kristof Van Assche, Sigrid Sterckx, Kurt Audenaert, Kenneth Chambaere, Free University of Brussels & Ghent University (VUB-Ugent) Room Gaspard de Maniban 8:00 am Workshop 3 Health risk management in the European Union Coordinator Nathalie De Grove Valdeyron O82 L'évaluation et la gestion des risques liés aux produits phytopharmaceutiques : quelle place pour le principe de précaution ? Quelle protection de la santé publique ? Nathalie De Grove Valdeyron Institut de recherche en droit européen, international et comparé (IRDEIC -Chaire Jean Monnet) Université Toulouse 1 Capitole O83 L’évaluation et la gestion des risques liés aux organismes génétiquement modifiés dans l’Union européenne Estelle BrossetProfesseure[i] Chaire Jean Monnet Aix Marseille Univ, France O84 Innovation in veterinary pharmaceuticals and antimicrobial resistance: assessment and management of public health risks in the European Union under the 'One Health' approach Stefania Negri Department of Legal Sciences (School of Law) - University of Salerno (UNISA) 10:30 am Session 12 (French) Chairpersons Berengère Legros (Université de Lille CRDP France) & Isabelle Poirot-Mazères (Université Toulouse1 Capitole France) O74 Pratiques et représentations des professionnels de santé autour de la loi Claeys-Léonetti sur la sédation en fin de vie .Bettina Couderc Alfonsina Faya Robles, Laurie-Anne Galiby, Emmanuelle Rial-Sebbag Trajectoires d'innovations en santé : enjeux bioéthiques et impact en santé publique (Biotherapeutics) Université Paul Sabatier-Toulouse III - UPS, IUCT Oncopole O75 L’injonction de soins, dernier né des soins pénalement ordonnés : innovation thérapeutique ou commande sociale ? P.A Delpla MCU-PH de Médecine Légale, Docteur en Droit, Expert et Médecin Coordonnateur O68 Le transhumanisme, une remise en question des droits fondamentaux par les innovations biotechnologiques ? Guylène Nicolas UMR 7268 ADES (Centre de droit de la santé) Aix Marseille Univ, CNRS, EFSAurélie Mahalatchimy UMR 7318 DICE CERIC, CNRS-Aix-Marseille Université - Université de Pau et des Pays de l’Adour - Université de Toulon et du Var O64 Innovation en génétique et renouvellement de la norme bioéthique Bérengère Legros Université de Lille (CRDP) O76 Intelligence artificielle et santé : à la recherche d’un statut juridique pour la prothèse Kamilia Bentaïeb – Doctorante en droit privé à l’Université Toulouse 1 Capitole – Centre de droit des affaires (CDA) 1:30 pm Workshop 11 Cross-border access to end-of-life services in EuropeCoordinators: Steven Lierman & Kristof Van Assche O110 Cross-border access to end-of-life services in Europe Steven Lierman Leuven Institute for Healthcare Policy KU Leuven (LIGB), University of Antwerp Kristof Van Assche Research Group Personal Rights and Property Rights, Faculty of Law, University of Antwerp Verhofstadt Monica Free University of Brussels & Ghent University (VUB-Ugent) O111 Cross-Border access to “sensitive” health care service in Europe Dr hab. dr Dobrochna Bach-Golecka Faculty of Law and Administration University of Warsaw 1:30 pm Session 8 Ethics Chairpersons: Mariela Deliverska (Medical University of Sofia Bulgaria) & Pierre Deschamps ( McGill University Canada) O46 The dangers for scientific progress and the rule of law of asphyxiating ethical considerations in a techno-centric world Pierre Deschamps C.M, Ad.E. Membre du groupe de recherche en santé et droit Université McGill Montréal Canada O47 EU values and ethics in health innovation & new technologies Markus Frischhut Management Center Innsbruck (MCI) O48 Religious-cultural Determinants of perception of Patient's Rights. Analysis based on the human rights standards.Ryszard Kozłowski Institute of Social Work (APS) Daria Bieńkowska Pomeranian University of Słupsk (APS) O49 Informed refusal of treatment in ethical and legal conflict with clinical pathway payment regulations Elisaveta Petrova-Geretto , Mariela Deliverska , Zlatitsa Petrova , Faculty of Public Health Sofia. O50 The ethics of cross-border biomedical data sharing: considering the application of Confucius’ moral philosophy Xiaojie Li, Yali Cong Center of medical ethics Pekin University China O51 Legal and ethical considerations in providing healthcare services to patients with rare diseases in Bulgaria (65) Mariela Deliverska Faculty of Public Health, Medical University Sofia Room Enjalbert 8:00 am Session 9 Innovation and health care Chairpersons Verena Stühlinger ( UMIT Innsbruck Autria) & Jacques Larrieu (Université de Toulouse1 Capitole France) O52 Directive 2011/24 Reimbursement rules and innovation in health care: a mismatch or an opportunity? Tomislav Sokol European Parliament (elected Member); Zagreb School of Economics and Management (EP; ZSEM) O53 Suicide Machines and Healthcare Innovation (133) Nataly Papadopoulou , Andreas Dimopoulos , Hui Yun Chan (University of Huddersfield) O54 EU Health Law and Policy – Shaping a Future Research Agenda Mary Guy Lancaster University O55 Innovative medical treatment in England: a “right to health” a “right to try” and a “right to fail”? Jean Mchale University of Birmingham [Birmingham] O56 Innovation, Rights/Obligations and Healthcare: The Physicians Legal Obligation to Report Potential Impaired Drivers in Romanian Medical Practice Maria Aluas Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca O57 New medical technology and evolution of legal approaches and principles Anne Kjersti Befring Inger-Johanne Sand University of Oslo O45 The role of legal regulation in the innovative health care Raimo Lahti University of Helsinki, Faculty of Law 3:30 pm Session 10 Medical Law Chairpersons: Dean Harris (University of North Carolina Chapel Hill, USA) & Athanasios Panagiotou (Aristotle University of Thessaloniki Greece) O58 Social rejection to vaccines Gustavo Merino (IDIVAL) O40 The Innovation of Self-Managed Medication Abortion: Increasing Access but Adding Legal Risks for Pregnant Women Dean Harris UNC Gillings School of Global Public Health (UNC-Chapel Hill) O60 Closing the gap: addressing health inequalities in Europe Brigit Toebes Department of Transboundary Legal Studies (RUG) O61 One-stream or Two-Stream? Legal and Policy Considerations for Maintaining a Separate Medical Cannabis Regulatory Framework in Canada Chelsea CoxSchulich School of Law, Dalhousie University O62 Redefining care in patient -physician relationship (56) Daria Bieńkowska Pomeranian University of Słupsk (APS) O63 Civil liability in health care: Modern trends in Russia Pospelova Svetlana,Kamenskaya Natalia A, Pavlova Yulia V. Sechenov First Moscow State Medical University (Sechenov University), Russia
POSTER SESSION (SALLE DES COLONNES) chairperson aurelie mahalatchimy (france) Posters of EAHL 7th Conference Jury : Renaud Bouvet (France) Nathalie de Grove Valdeyron (France) Yann Joly (Canada) Aurelie Mahalatchimy (France) Stefania Negri (Italie) Judit Sandor (Hungary). P01 Bodily Products: Waste, Ownership and Harm Neil Maddox National University of Ireland Maynooth (NUIM) P02 Lawsuits against Environmental Health Risks Michael Ganner Leopold Franzens Universität Innsbruck - University of Innsbruck P03 Legal Status of the Fetus as a Patient, Participant of Legal Relations in the Field of Health Care Provision Roksolana Kazimirska, Iryna Senyuta Bar Association "MedLex" Danylo Halytsky Lviv National Medical University P04 The qualification of medical treatments and possible consequences of a lacking informed consent Javier Jimenez Hoertnagl Leopold Franzens Universität Innsbruck – University of Innsbruck P05 Legal Regulation of Assisted Reproductive Technologies in Georgia :The Gap between Law and Practice Mari Rodonaia, Gulnara Shelia Tsereteli State University P06 Legal dimension of the traditional and complementary medicine practices in Turkey Zeynep Reva Istanbul Medeniyet University Medicine Law Department - Doctorate Program (Istanbul Medeniyet University) P07 Liability for nosocomial infections Thomas Pixner University of Innsbruck, Austria - Department of Civil Law .Doctoral Programme Medical Law and Health Care P08 Interoperability in e-health: what role for European Union law? Nahela El Biad CERIC, UMR DICE 7318 (CERIC) Aix Marseille Université (Aix-en-Provence)
P09 What is the industries' involvement in the adoption of the European guideline on quality, preclinical and clinical aspects of gene therapy medicinal products? Quentin Longin , Benoit Immordino , Pauline Heyries , Alix Leenhardt , Véronique Andrieu , Aurélie Mahalatchimy –UMR 7318 Droits International, Comparé et Européen CERIC (UMR 7318 DICE CERIC) Faculté de Pharmacie Aix-Marseille Université. P 10 Acceptation of e-health connected objects Camille Magne Floriane Stephan, Julien Duguet .INSEEC SCHOOL OF BUSINESS AND ECONOMICS, Bordeaux, France ; INSERM 1027 P11 L'utilisation des informations génétiques non recherchées, révélées par le séquençage de nouvelle génération, en oncologie pédiatrique Sandra Le Tirant , Sophie Julia Emmanuelle Rial-Sebbag UMR 1027 INSERM, Université Toulouse III – Paul Sabatier ; Service de Génétique médicale, Hôpital Purpan, CHU de Toulouse CHU Toulouse P12 The subtle European definition of gene therapy medicinal products: Navigating the borderline scenarios Aurélie Mahalatchimy , Nicolas Sicart, Lucile Zani , Maël Stenou UMR 7318 DICE CERIC Aix Marseille Université ; Faculté de Pharmacie, Aix-Marseille Université P13 Vers une gouvernance internationale et européenne de l'intelligence artificielle dans le domaine de la santé Anthéa Serafin , Emmanuelle Rial-Sebbag UMR 1027 INSERM, Université Toulouse III – Paul Sabatier; LEASP - UMR 1027. P 14 Provision of services in psychiatric and residential institutions in Serbia in line with human rights standards - insights from qualitative research and proposals for legislative reforms Marta Sjenicic 1, Hajrija Mujovic, Isidora Jaric, Milos Milenkovic, Marko Milenkovic Institut of Social Sciences (ISS) P15 Legal and Ethical Issues of Human Germline Editing Gauthier Chassang Anastasia Constantin , Bettina Couderc, Emmanuelle Rial-Sebbag Inserm UMR 1027 – UPS Toulouse 3 ; Institut Claudius Regaud – IUCT-Oncopole P16 Medicine professionals' health. Health care programs. Milagros Estrada Maartinez University of Cantabria P17 Paternity DNA Testing: how to find right balance in complex situations Vugar Mammadov Gediminas Sagatys WAML, Law Faculty of Baku State University (WAML, BSU) ; Supreme Court of Lithuania, Vilnius, Lithuania P18 Level of socio-economic wellbeing as an ethical aspect in health care system Vugar Mammadov, Lala Jafarova , Leyli Mammadova WAML, Law Faculty of Baku State University ;Azerbaijan Medical University (AMU) ; Institute of Law and Human Rights, Azerbaijan National Academy of Sciences (NASA) P19 Innovation and health care : The case of prenatal medicine Dr hab. dr Dobrochna Bach-Golecka Faculty of Law and AdministrationUniversity of Warsaw
P20 How innovative homeopathy can be? Katarzyna Miaskowska-Daszkiewicz The John Paul II Catholic University of Lublin Cancelled
P21 Extramural healthcare for patients suffering from Korsakoff's syndrome: a new challenge Susanne Van Den Hooff Amsterdam University of Applied Sciences (HvA) P22 Research and development of a new technology for genome-wide DNA methylation profiling rises many ethical and legal questions Marjolein Timmers 1, Ernst Hulst : Erasmus School of Health Policy and Management |Rotterdam] (ESHPM) P23 A new generation of medicines needs new generation compounding. Tina Tititma Researcher PhD Talinn University of Technology EAHL National Contect Estonia P24 Personalized Medicine in Europe, Prompting Biopolitical Foresights on Stratified Methods Céline Aludaat-Dujardin Faculty of law Helsinki P25 L'innovation dans les maladies rares : quel accès pour les patients en France ? Florence Taboulet , Bourdoncle Marion , Juillard-Condat Université Paul Sabatier Toulouse 3 - INSERM UMR 1027 P 26 (additionnal list of abstracts) Integrating 9 digital simulators to the initial training of 8000 nursing students in the region of Nouvelle-Aquitaine (France) Decormeille G. Brun G. Huet N , Leleu J, Geeraerts T. Université Toulouse Jean Jaurès laboratoire CLLE-LTC, UMR 5263 CNRS Simforhealth, Bordeaux Institut Toulousain de Simulation en Santé (itSimS, CHU de Toulouse) P27 (additionnal list of abstracts) The enforceability of judgements of foreign disciplinary courts in cases of disciplinary responsability of the physicians. The Polish case. Pr Monika Urbaniak (Poznan University of Medical Sciences , Poland) Oral presentations changed to posters P28 (abstract O20) Witness Immunity in the Field of Health Care: Balance between Public and Private Interest Iryna Senyuta Danylo Halytsky Lviv National Medical University P29 (abstract O36) Information rights during medical experiments Khrystyna Tereshko Danylo Halytsky Lviv National Medical University Posters of young scientists and PhD Jury: Markus Frischhut (Management Center Innsbruck -MCI) Titti Mattsson Faculty of Law Health Law Research Centre, Lund University PHD P1 Shaping new legal strategies under Directive 2011/24/EU to accommodate national divergence in the protection of classical, individual patients’ rights Bongers Lisette (Maatricht University) PHD P2 European reference networks: a clear added value for patients' health Bories Claire PHD P3 A Human Right-Based Approach to Health Technology Assessment (HTA) Bottini Filho Luciano Pentonville Road GB PHD P4 Big Data and governmental decision-making in healthcare: The role of the principles of good administration, regulation and legislation Miet Caes (PhD researcher at the Leuven Institute for Healthcare Policy, KU Leuven) PHD P 5 Therapeutic privilege – the reminiscence of paternalism defying the principle of patient's autonomy or the necessary exception? A comparative study of the figure in Spanish and Polish law Gesinska Marta Universidad de la Rioja (ES) PHD P6 Doctors legal position in complaint – and supervision cases Logstrup Kathrine Sidsel (Aarus BSS Denmark) PHD P7 Guidelines, good practices and best clinical health practices: valuable guidance for physicians and judges? Mazzariol Brenno (La Spienza Univeristy of Rome) PHD P8 Le programme européen Priority Medicines (PRIME): un réel accélérateur de l’accès des patients à des médicaments sûrs et innovants ? Noëllie Roudière Droit pharmaceutique et Economie de la santé- Inserm UMR 1027- Unive rsité Toulouse III PHD P9 The use of novel mobile applications in mental healthcare: a consideration of its medical, legal and ethical implications. Michael Z Tai Furness general hospital UK PHD P10 La télépharmacie : effet de mode ou renforcement du rôle du pharmacien ? Marie-Joseph Vianney Centre de droit de la santé de l’Université Aix-Marseille III.
|